
Offering help is easy. Asking for hep is hard.
Help make future vaccines possible.
An estimated 58 million people, including 250,000+ Canadians, are living with hepatitis C.

The problem
Even with curative treatments, the number of people affected by hepatitis C has not decreased. Vaccines could make a huge difference in global hepatitis C elimination efforts.

The bad news
Diagnosis and treatment reaches a minority of people living with chronic hepatitis C, killing hundreds of thousands of people every year.
Testing vaccine candidates for hepatitis C using naturally occurring infections has been slow and expensive.

The good news
Hepatitis C infection has no short term health impacts and is curable with a well tolerated treatment regimen making a controlled human infection model (CHIM) for this disease possible.
A CHIM would significantly speed hepatitis C vaccine development.
Help make a vaccine possible.
Healthy males aged 18 to 40 in the greater Toronto area have a unique opportunity to take part in a compensated clinical study. This study is one step in creating a controlled human infection model (CHIM), which will be key to testing future hepatitis C vaccines. Recruitment will likely begin in 2025.
The study will be outpatient, requiring clinic visits but not overnight stays.
Participants will be compensated for their participation, including reimbursements for transportation costs.

What’s involved?
Interested participants will complete informed consent and medical screening processes before officially enrolled in the study. This website is to express interest in learning more about the study.
Study participants are infected with hepatitis C through a blood sample that has been confirmed to be free of other diseases. In the weeks following, participants will be required to visit the clinic for clinical procedures. These include:
- Plasmapheresis, aka plasma exchange
- Blood draws
- Non-invasive liver scans
- Treatment in the form of daily direct-acting antiviral (DAA) pills
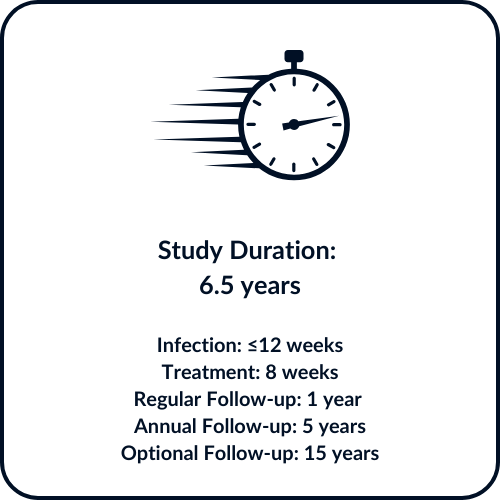
Active participation in the study is approximately 12 weeks, followed by 8 weeks of treatment. 4 follow-up visits will occur in the year after treatment with yearly follow-ups will continue for an addition 5 years.
You may withdraw from this study at any time without providing a reason.
Frequently Asked Questions
How does it work?
Adult volunteers are deliberately infected with hepatitis C using plasmapheresis. The body’s responses are monitored through blood draws and liver scans. After the initial infection period, participants are treated and return for intermittent follow-up visits.
Study participants can elect to leave the study at any time.
Could I infect others on accident?
Hepatitis C is spread through direct, blood-to-blood contact, which is very hard to do in daily life.
You and your partner should avoid sharing items that could have trace amounts of blood on them, like toothbrushes, razors, earrings or piercing jewelry, and nail clippers. Sharing plates and silverware does not put you at risk.
While hepatitis C cannot be shared through contact like hugging or kissing, it can be transmitted sexually and condoms should be used.
What are the risks?
Approximately 80% of people with hepatitis C have no symptoms before liver issues appear decades after infection. The symptoms are generally mild and intermittent. Short-term infection, like in a human challenge study, would not increase risk of liver problems in the future.
Common symptoms include fever, fatigue, nausea, vomiting, loss of appetitie, dark urine, light stool, abdominal pain, and jaundice.
Similar to other clinical research studies, HCTs require regulatory and ethic board approval and will be followed throughout the duration of the study.
The two Nobel Prize-winning scientists who discovered hepatitis C, along with 119 others, published a letter of support for hep C human challenge trials.
Brought to you by

